Unlocking the Secrets of CAS9 Mutations: Advanced Molecular Dynamics Simulations by LambasLab

In the fast-evolving fields of pharmaceuticals and biology, understanding the behavior of proteins is paramount. At LambasLab, we are at the forefront of this research, leveraging cutting-edge molecular dynamics simulations and quantum-chemical calculations to provide unparalleled insights into protein behavior and drug interactions. Our recent study on mutant variants of the CAS9 protein exemplifies our expertise and the advanced capabilities we offer to researchers and developers in the biopharmaceutical industry.
Revealing the Dynamics of CAS9 Mutations
The CAS9 protein, a cornerstone of genome editing technology, holds immense potential for therapeutic applications. However, mutations within CAS9 can significantly alter its behavior, impacting its efficacy and safety. Our team at LambasLab embarked on an in-depth investigation of various mutant variants of CAS9 to decode these complex dynamics.
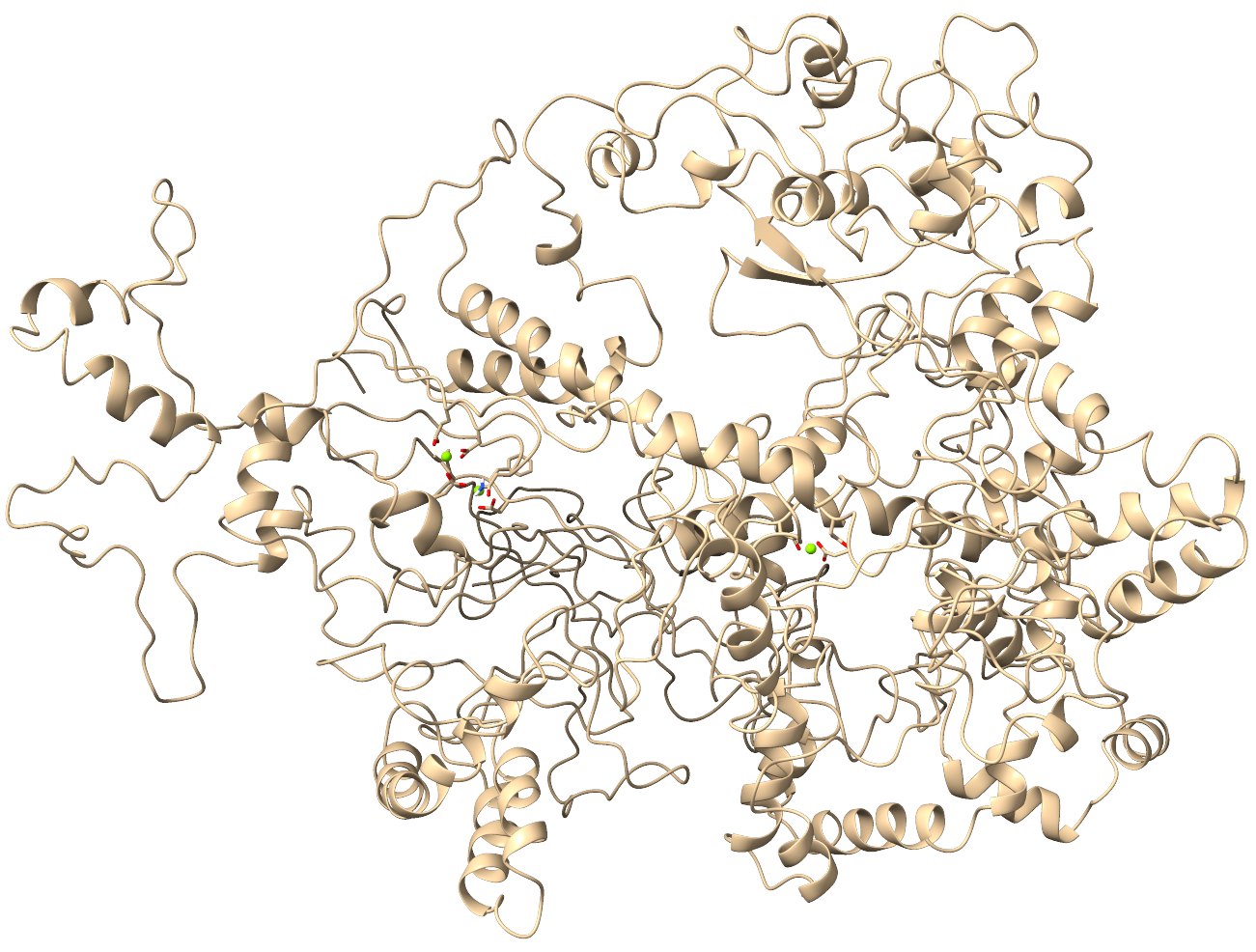
Enhancing Experimental Results
Our service enhance experimental studies with molecular dynamics simulations. Experimentally, our clients assessed the enzymatic activity of several CAS9 mutants, focusing on their genome-editing capabilities. They suggest extended set of CAS9 mutants for MD simulation. Our MD simulations provided a detailed picture of how these mutations influence the protein's conformational behavior at the molecular level.
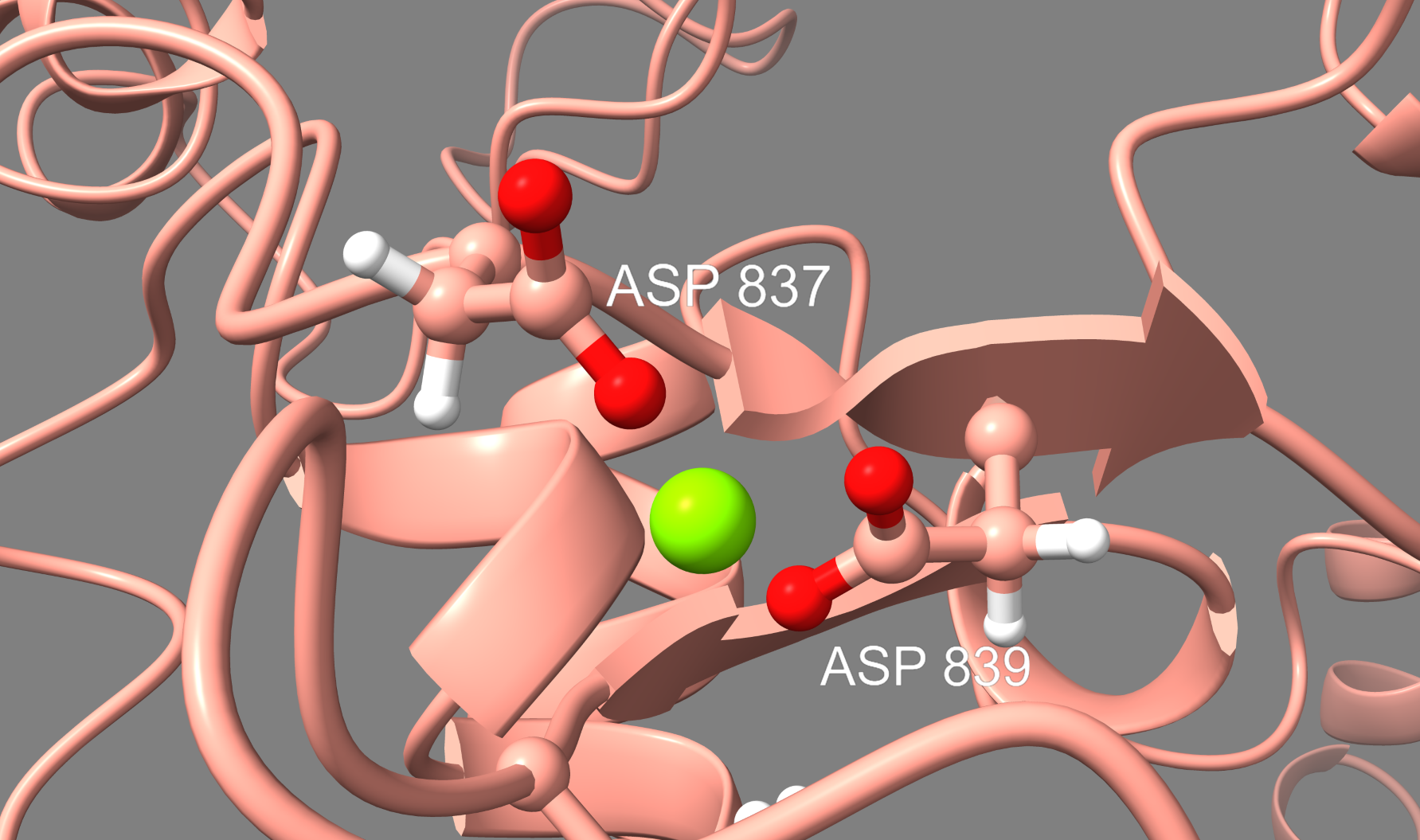
Key Findings: Mobility and Conformational Changes
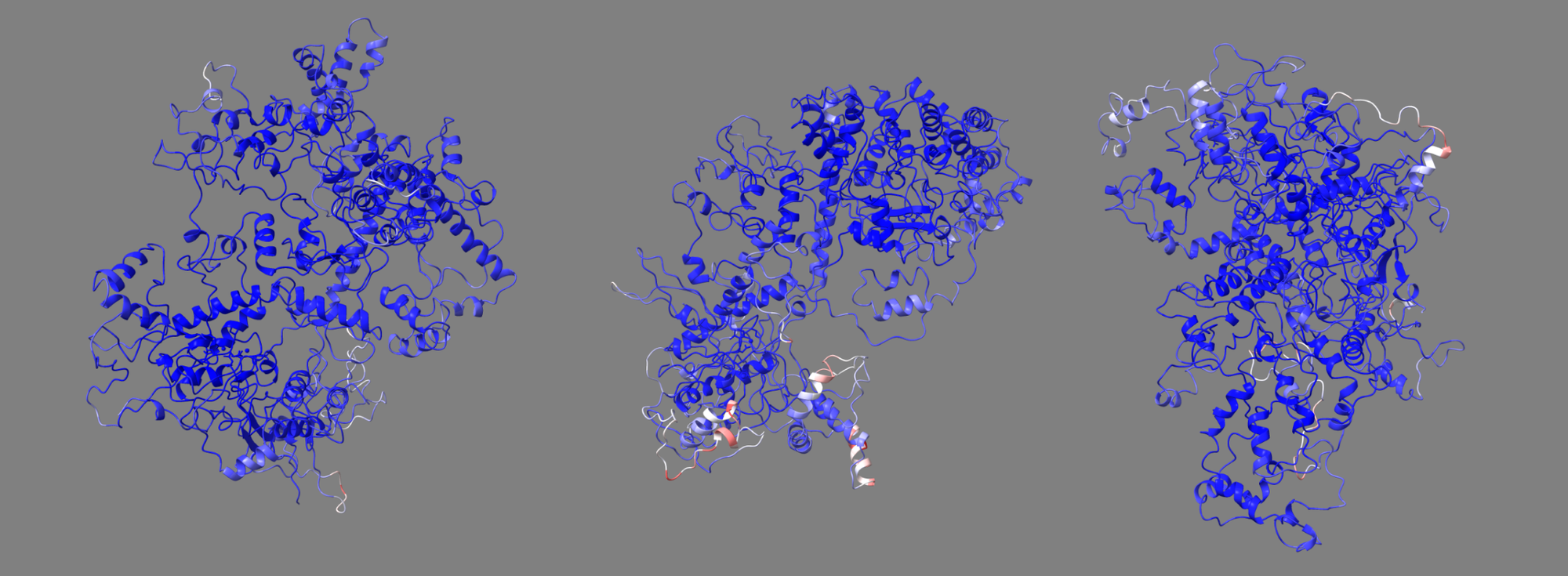
Using root mean square fluctuation (RMSF) maps, we pinpointed regions within the CAS9 apoprotein that exhibited increased or decreased mobility due to mutations. These insights are crucial for understanding how structural changes affect the protein's function and stability.
Furthermore, our analysis of root mean square deviation (RMSD) graphs revealed which mutations render the CAS9 apoprotein more conformationally flexible or rigid. This information is vital for predicting the protein's behavior under different conditions and designing more effective genome-editing tools.
Compaction and Decompaction Insights
Our study also included an analysis of gyration graphs, which illustrated how different mutant structures tend towards compaction or decompaction. These findings provide valuable clues about the structural stability and potential aggregation properties of the mutants, which are critical factors in drug design and therapeutic applications.
Partner with LambasLab for Cutting-Edge Research
At LambasLab, we are dedicated to advancing the frontiers of molecular dynamics and quantum-chemical research. Our expertise in simulating and analyzing complex biological systems positions us as a trusted partner for researchers and developers aiming to unlock the full potential of their projects.
Whether you are investigating protein mutations, studying drug-receptor interactions, or exploring new therapeutic avenues, LambasLab offers the advanced computational tools and scientific expertise you need to succeed. Partner with us to elevate your research and achieve groundbreaking results.